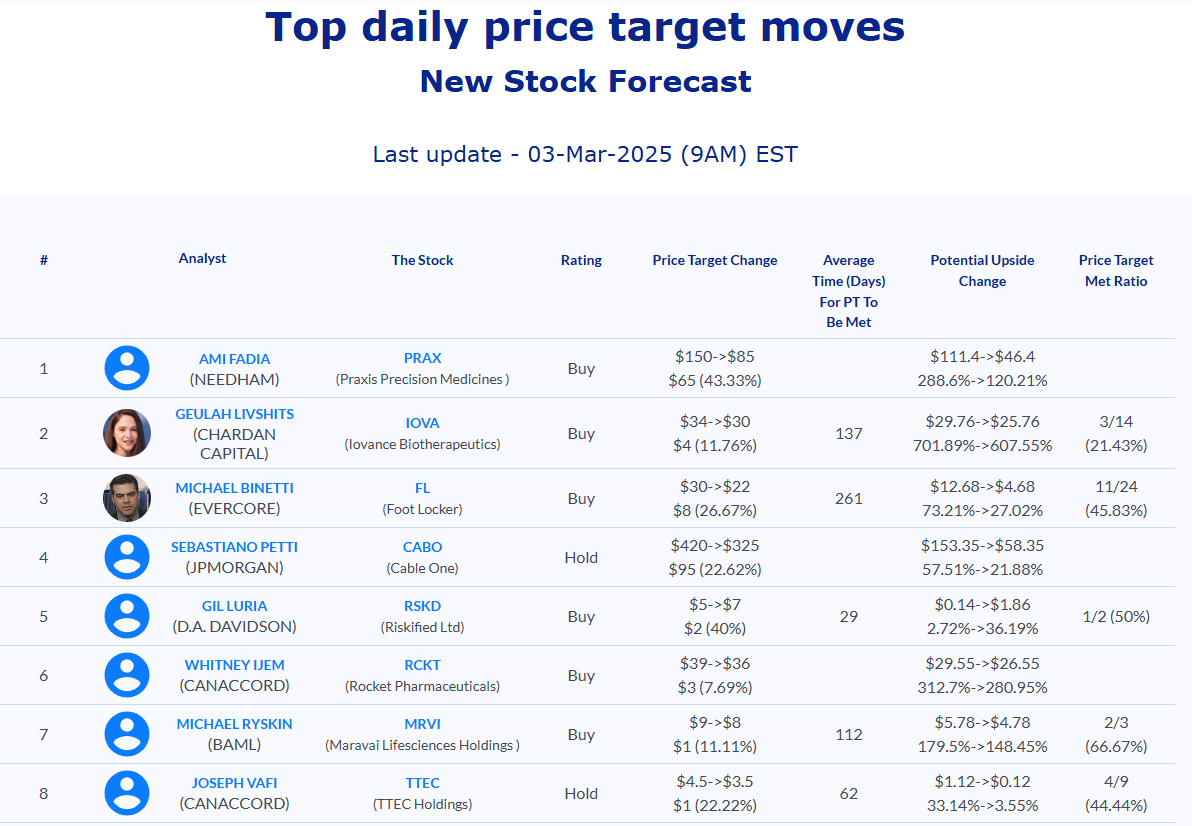
Selected stock price target news of the day - March 3rd, 2025
By: Matthew Otto
Celldex Reports Q4 2024 Financial Results and Provides Clinical Pipeline Update
Celldex Therapeutics reported a fourth-quarter 2024 earnings per share (EPS) of ($0.71), missing analyst estimates of ($0.69) by $0.02. Revenue for the quarter reached $1.18 million, exceeding the consensus estimate of $825.56 thousand.
Celldex also provided an update on its clinical pipeline, highlighting ongoing Phase 3 studies of barzolvolimab in chronic spontaneous urticaria (CSU) and plans to initiate a Phase 3 program in chronic inducible urticaria (CIndU) in 2025. Additionally, Celldex completed enrollment in its Phase 2 study of barzolvolimab in eosinophilic esophagitis (EoE) and continues enrolling patients in Phase 2 studies for prurigo nodularis (PN) and atopic dermatitis (AD). Celldex also launched a Phase 1 study of its first bispecific antibody, CDX-622, targeting stem cell factor (SCF) and thymic stromal lymphopoietin (TSLP) for inflammatory diseases, with key data readouts anticipated in 2025.
Financially, Celldex ended 2024 with $725.3 million in cash, cash equivalents, and marketable securities, down from $756.0 million at the end of the third quarter, primarily due to $32.5 million in cash used for operating activities. Celldex reported total revenue of $7.0 million for the full year, with research and development (R&D) expenses increasing to $46.9 million in Q4 and $163.6 million for the year, driven by clinical trial and personnel costs. General and administrative (G&A) expenses rose to $10.3 million in Q4 and $38.5 million for 2024 due to higher stock-based compensation and commercial planning expenses.
Celldex reported a net loss of $47.1 million ($0.71 per share) for Q4 and $157.9 million ($2.45 per share) for the full year, compared to net losses of $43.3 million ($0.83 per share) in Q4 2023 and $141.4 million ($2.92 per share) for 2023.
Analyst Reiterates Rating and Target Amid Progress and Q4 Results
- HC Wainwright & Co. analyst Joseph Pantginis reiterated with a Buy rating and a $80 price target.
Which Analyst has the best track record to show on CLDX?
Analyst Kristen Kluska (CANTOR FITZGERALD) currently has the highest performing score on CDLX with 4/18 (22.22%) price target fulfillment ratio. Her price targets carry an average of $41.49 (162.64%) potential upside. Celldex Therapeutics stock price reaches these price targets on average within 62 days.
Akero Therapeutics Reports Positive Clinical Progress and Financial Results
Akero Therapeutics reported advancements in its clinical programs, including unprecedented findings from its Phase 2b SYMMETRY study. The study demonstrated a reversal of compensated cirrhosis (F4) in 39% of patients treated with 50mg efruxifermin (EFX) for 96 weeks, compared to 15% in the placebo group. Additionally, an ITT analysis showed fibrosis improvement in 29% of treated patients versus 12% in the placebo group.
Financially, Akero ended 2024 with $797.8 million in cash, cash equivalents, and marketable securities, following a $402.5 million stock offering in January 2025. Research and development expenses rose to $69.3 million for Q4 2024 and $247.5 million for the full year, up from $53.4 million and $141.8 million in 2023, primarily due to increased costs associated with the Phase 2b SYMMETRY and Phase 3 SYNCHRONY studies. General and administrative expenses increased to $37.9 million in 2024 from $31.1 million in 2023.
Analyst Raises Price Target Following Updates
- HC Wainwright & Co. analyst Ed Arce maintained a Buy rating and raised the price target from $72 to $75.
Which Analyst has the best track record to show on AKRO?
Analyst Ed Arce (HC WAINWRIGHT) currently has the highest performing score on AKRO with 12/24 (50%) price target fulfillment ratio. His price targets carry an average of $20.29 (39.24%) potential upside. Akero Therapeutics stock price reaches these price targets on average within 266 days.
Praxis Precision Medicines Provides Updates on Clinical Programs and Financial Performance
Praxis Precision Medicines has provided an update on its Essential3 program for ulixacaltamide in essential tremor, along with progress in its broader pipeline and financial performance. The Independent Data Monitoring Committee (IDMC) recommended stopping Study 1 of the Essential3 program for futility, as interim results indicated the study was unlikely to meet its primary efficacy endpoint. However, Praxis will continue both Study 1 and Study 2 to completion, with topline results expected in Q3 2025.
Financially, Praxis reported $469.5 million in cash, cash equivalents, and marketable securities as of December 31, 2024, compared to $81.3 million a year prior. Praxis recognized $8.6 million in collaboration revenue for the full year, mainly from its agreement with UCB. Research and development expenses rose to $152.4 million in 2024, up from $86.8 million in 2023, while general and administrative expenses increased to $56.3 million from $42.1 million. Praxis reported a net loss of $182.8 million for 2024, compared to $123.3 million in 2023.
Analysts Maintain Ratings While Adjusting Price Targets
- Baird analyst Joel Beatty maintained an Outperform rating, yet lowered the price target from $117 to $73.
- Needham analyst Ami Fadia continued a Buy rating but reduced the price target from $150 to $85.
- HC Wainwright & Co. analyst Douglas Tsao upheld a Buy rating while adjusting the price target downward from $120 to $105.
Which Analyst has the best track record to show on PRAX?
Analyst Laura Chico (WEDBUSH) currently has the highest performing score on PRAX with 11/14 (78.57%) price target fulfillment ratio. Her price targets carry an average of $-4.38 (-7.91%) potential downside. Praxis Precision Medicines stock price reaches these price targets on average within 105 days.
Daily stock Analysts Top Price Moves Snapshot