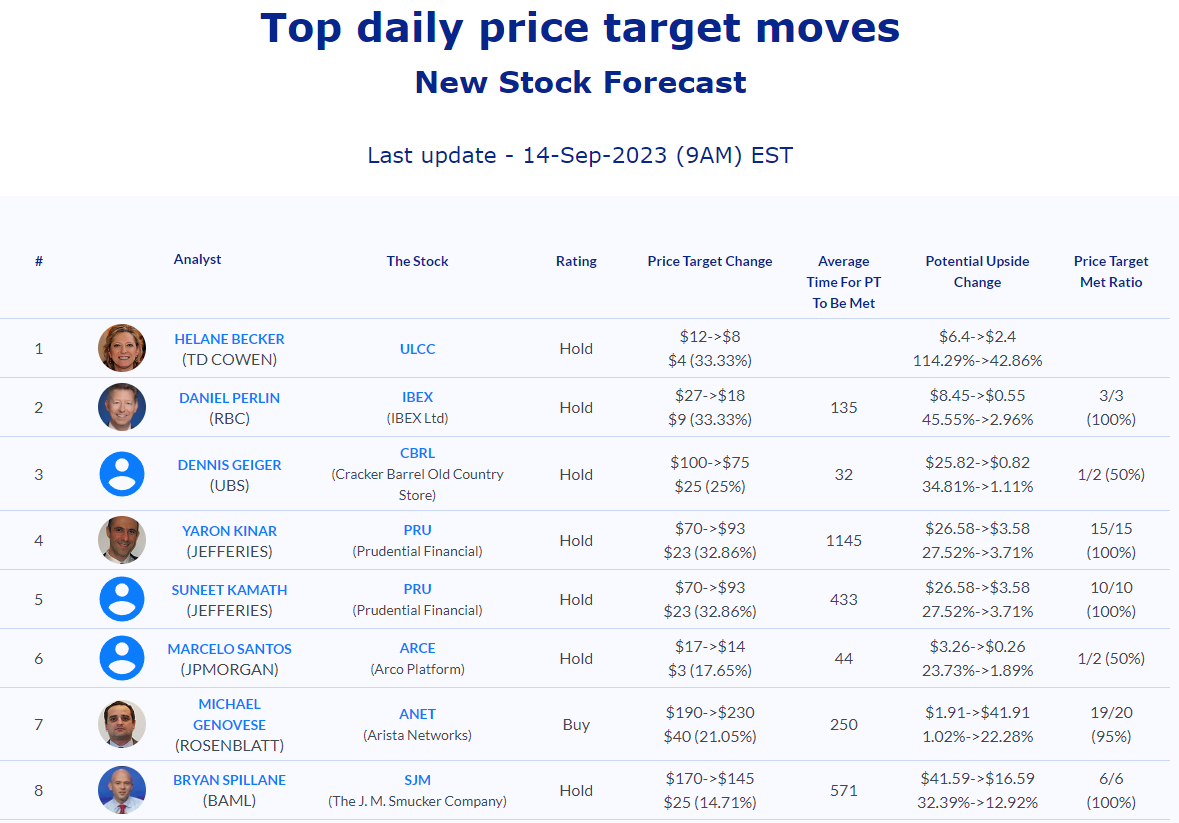
Selected stock price target news of the day - September 14, 2023
By: Matthew Otto
Semtech Q2 Fiscal Year 2024 Earnings Call Highlights Growth and Financial Resilience
Semtech Corporation has reported its financial results for the second quarter of fiscal year 2024. The company achieved net sales of $238.4 million during this period, in line with their guidance and reflecting a 1% sequential increase and a robust 14% year-over-year growth. Their gross margin stood at 49.6%, surpassing the expected range, and earnings per share reached $0.11, exceeding the high end of their guidance.
In terms of geographic distribution, Semtech’s shipments were well-balanced, with North America, China, Europe, and the Rest of the World accounting for 24%, 29%, 14%, and 33% of net sales, respectively. Additionally, the acquisition of Sierra Wireless has influenced the company’s geographic mix, particularly strengthening their presence in North America and Europe. Looking ahead, Semtech projects an outlook for the third quarter of fiscal year 2024, with an expected net sales range of $190 million to $210 million.
Analyst Actions Diverge on Semtech as Sentiments Vary
- Benchmark analyst Cody Acree downgraded from Buy to Hold.
- Needham analyst Quinn Bolton reiterated a Buy rating and a $35 price target.
- Summit Insights Group analyst Kinngai Chan upgraded from Hold to Buy.
- Susquehanna analyst Christopher Rolland upgraded from Neutral to Positive and announced a $30 price target.
Analyst Christopher Rolland (SUSQUEHANNA) currently has the highest performing score on SMTC with 9/12 (75%) price target fulfillment ratio. His price targets carry an average of $4.14 (10.14%) potential upside. Semtech stock price reaches these price targets on average within 43 days.
Neurocrine Biosciences’ INGREZZA® Oral Granules NDA Accepted by FDA
Neurocrine Biosciences has achieved a significant milestone in expanding treatment options for individuals with movement disorders. The U.S. Food and Drug Administration (FDA) has recently accepted the New Drug Application (NDA) for INGREZZA® (valbenazine) oral granules, a novel sprinkle form of INGREZZA® capsules. This development aims to offer an alternative administration method for patients who may have difficulty swallowing traditional capsules, particularly those with conditions like tardive dyskinesia and chorea associated with Huntington’s disease. The FDA has set a target action date of April 30, 2024, for the potential approval of INGREZZA oral granules.
The INGREZZA oral granules are available in three dosages: 40 mg, 60 mg, and 80 mg, with the capsules designed to be opened and sprinkled on soft foods for easier administration. This innovation addresses a crucial need among patients who experience dysphagia, difficulty swallowing, due to their conditions. Currently, INGREZZA stands as the sole once-daily treatment option, delivered in a single capsule, for adults dealing with tardive dyskinesia and chorea associated with Huntington’s disease. Neurocrine Biosciences’ pursuit of improving patient lives through innovation reflects the ongoing progress in neuropharmacology and the drive to provide more accessible treatment options for those affected by these complex disorders.
Analysts Offer Varied Recommendations and Price Targets
- Canaccord Genuity analyst Sumant Kulkarni maintained a Buy rating and raised the price target from $132 to $144.
- Mizuho analyst Uy Ear reiterated a Neutral rating and a $113 price target.
- Wedbush analyst Laura Chico maintained an Outperform rating and increased the price target from $120 to $137.
Analyst Charles Duncan (CANTOR FITZGERALD) currently has the highest performing score on NBIX with 27/35 (77.14%) price target fulfillment ratio. His price targets carry an average of $34.85 (67.01%) potential upside. Neurocrine Biosciences stock price reaches these price targets on average within 505 days.
Alnylam’s Patisiran Receives Positive Review for Potential Cardiomyopathy Treatment in ATTR Amyloidosis
Alnylam Pharmaceuticals has released an update following the meeting of the U.S. Food and Drug Administration’s (FDA) Cardiovascular and Renal Drugs Advisory Committee (CRDAC) concerning the supplemental New Drug Application (sNDA) for patisiran, an investigational RNAi therapeutic designed to address cardiomyopathy associated with transthyretin-mediated (ATTR) amyloidosis. During the meeting, the CRDAC voted 9:3 in favor of the benefits of patisiran outweighing its associated risks for this specific indication. This development represents a significant step forward in offering a new and innovative treatment option for patients grappling with this rare and debilitating condition, given the limited current treatment choices available.
ATTR amyloidosis is a severe ailment resulting from the accumulation of misfolded transthyretin (TTR) proteins in the form of amyloid deposits in different parts of the body, including the heart. This accumulation leads to cardiomyopathy and eventual heart failure. Notably, patisiran, also known as ONPATTRO®, has already received FDA approval for addressing the polyneuropathy of hereditary ATTR amyloidosis in adults. The FDA is scheduled to make a determination regarding the potential expanded indication for patisiran on October 8, 2023, in accordance with the Prescription Drug User Fee Act.
Analysts Offer Differing Views on Alnylam with Reiterations
- Cantor Fitzgerald analyst Olivia Brayer reiterated Neutral rating and a $190 price target.
- Needham analyst Joseph Stringer maintained a Buy rating and a $240 price target.
Analyst Keay Nakae (CHARDAN CAPITAL) currently has the highest performing score on ALNY with 9/12 (75%) price target fulfillment ratio. Her price targets carry an average of $45.83 (47.46%) potential upside. Alnylam Pharmaceuticals stock price reaches these price targets on average within 396 days
Daily stock Analysts Top Price Moves Snapshot